
Testing new wearable technology to monitor patients after surgery
Hamilton Health Sciences (HHS) currently has a successful program that uses remote monitoring technology to support patients at home after surgery. However, researchers at Population Health Research Institute (PHRI) of HHS and McMaster University are looking to test a new remote home monitoring device that continuously tracks patients’ vital signs so they can be monitored around the clock in hospital and at home to earlier detect signs of post-surgical complications.
Continuously tracking vital signs
The new continuous vital signs monitoring device is called VitalitiTM by Ontario-based health-care technology company, Cloud DX. VitalitiTM tracks the patient’s ECG (the heart’s electrical activity), heart rate, pulse rate, respiration rate, temperature, blood pressure and oxygen saturation. This provides the health-care team with continuous patient data that will be used in future studies to detect subtle signs of deterioration, such as signs of infection leading to sepsis, and allow the health-care team to intervene early.
As a first step, the VitalitiTM device needs to be evaluated for accuracy. Then, it’s brought to patients to get their feedback. These are extremely important steps that need to be done before using the device in large studies with thousands of people.
What’s the VERDICT?
This preliminary validation study is called VERDICT-2 and is led by HHS emergency department nurse, McMaster doctoral student and PHRI research fellow, Carley Ouellette.
To test the accuracy of the device, Ouellette will recruit 35 healthy individuals to spend a few hours wearing the VitalitiTM device while also being connected to the individual medical equipment that monitors the same vitals. Individuals will be required to do different movements like walking, sitting, lying down, and moving their head around, to see if it impacts the device’s ability to record data.
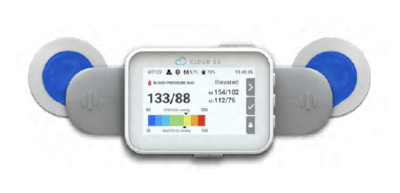
This new wearable device adheres to the patient’s chest so it can continuously track their vitals while in hospital and at home.
Then, to test if VitalitiTM can be worn long-term, and to understand the patient experience, Ouellette will recruit a similar number of surgical patients to wear the device at home after discharge for three days. She will also talk to the patients’ caregivers, nurses and doctors to see what they think about the monitoring technology. The device is not turned on, as that’s what the large-scale study will be for, it’s just to see if patients have any concerns with wearing it.
Ouellette and team are about to launch VERDICT-2 within the next few weeks. This is the second validation study for VitalitiTM. The first took place in 2021, with results published in February 2022.
Importance of testing
“In VERDICT-1, we found that while the device was accurate with tracking blood pressure, the design needed to be changed. It was worn around the neck, which might not be comfortable to wear long-term,” says Ouellette. “Since then, the device has been redesigned and is now worn on the chest.”

The Vitaliti device originally wrapped around the neck but has since been redesigned as a result of patient feedback.
The new version of VitalitiTM is lighter and smaller. It no longer wraps around the neck and now adheres to the patient’s chest. As with the original design, a piece extends to the earlobe, in order to get the ECG reading.
“This just shows the importance of doing an accuracy and user-test study,” says Ouellette. “While we thought the original design was a unique way to be non-intrusive, in reality, that didn’t end up being the case.”
She continues by saying “It has been a great partnership working with Cloud DX to test and implement innovative technology that has the potential to make a real clinical difference with patients who are at risk for serious postoperative complications. This is something the whole research team is excited about.”
This work is a part of Ouellette’s doctoral research for which she received funding through the CIHR 2022 Vanier Scholarship. It is a precursor to the upcoming large observational study called VISION-2, led by PHRI scientists Drs. PJ Devereaux and Mike McGillion. It will include 20,000 surgical patients around the world who will wear the Vitaliti™ device after surgery.




