
HHS Medical Hero: Lubinda Liswaniso
Helping future generations through research
Hemophilia runs in Lubinda Liswaniso’s family. The Milton teen was born with the rare genetic disorder where blood does not clot properly. He is taking part in an international clinical trial at Hamilton Health Sciences (HHS) looking at the safety and effectiveness of a new drug for patients with hemophilia, and whether it will provide better protection from bleeding with a less invasive treatment. The product being tested is not yet approved and any benefits listed are potential benefits only.
“I wanted to help with research into hemophilia.”
Lubinda is also participating in a sub-study on joint health for hemophilia patients that’s attached to the clinical trial.
“I wanted to help with research into hemophilia,” says Lubinda, 17, who was invited to take part by Dr. Anthony Chan, an HHS pediatric hematologist and the clinical trial’s local principal investigator.
“I’m hopeful that medication and treatment can be improved through research,” says Lubinda. “My wish is that through research, we eventually get to the point where patients with hemophilia can go months between treatments instead of days. It’s nice to have needles less often.”
Partners in research
Clinical trials are leading to better outcomes for patients in Hamilton, across Canada and around the world, and would not be possible without patients like Lubinda who volunteer to take part. Lubinda has hemophilia A, caused by missing or defective clotting protein in the blood called factor VIII (eight). The new drug being tested focuses on hemophilia A patients ages 12 and older. A clinical trial has just been opened for patients with hemophilia under six years old.

Lubinda Liswaniso, centre, with Dr. Anthony Chan and physiotherapist Karen Strike.
Hemophilia A is treated with factor replacement therapy, where factor VIII concentrates are injected into a patient’s vein to prevent or control bleeding by maintaining protective levels. Patients are taught to do this themselves, from the comfort of their own home. Parents also learn, so they can help children and teens not yet ready to inject medication for themselves. With this new medication, Lubinda only needs an injection once a week instead of three times a week.
“It’s nice to have needles less often.”
Also, accessing a vein is challenging for Lubinda, so he welcomes the opportunity through this study to infuse less often.
The domino effect
To understand how the body creates clots to stop bleeding, picture dominos toppling to create a chain reaction. “Imagine that each domino is a different factor and patients with hemophilia A are missing a domino representing factor VIII,” says Karen Strike, a physiotherapist with the bleeding disorders clinic at HHS’ McMaster Children’s Hospital. She’s also sub-investigator for the joint health study using ultrasound to examine patients’ joints. “When the clotting process starts, the dominos begin tipping.”
“When the clotting process starts, the dominos begin tipping.”
But if the domino representing factor VIII is missing or reduced, the chain reaction becomes impaired and this can result in inadequate clotting.”
With the medication being tested, the missing `domino factor VIII’ is replaced with a synthetic product so blood coagulation can continue to form a clot. “In other words, the dominoes continue to tumble,” says Strike. “What’s unique about this product is that the domino will also stick around a little longer, giving the patient better protection.”
For patients like Lubinda, this means one weekly injection instead of a needle three times a week. “Lubinda can now get once a week infusion with a trough level — the lowest concentration of the drug in the patient’s bloodstream — higher than when he was getting it three times a week,” says Chan. “As a result, people with hemophilia can enjoy more activities with lower risk of bleeding.”
The clinical trial is in phase three – the final phase before approval. A phase three clinical trial evaluates how the new medication works compared to existing medications.
Joint health
For patients with hemophilia, approximately 90 per cent of bleeding episodes occur in their joints and muscles, with knees, elbows and ankles being the most common joint bleeds.
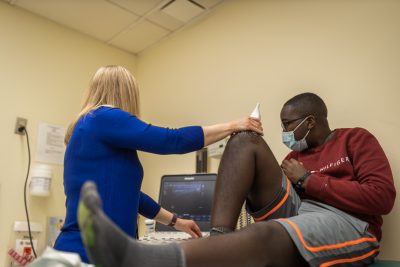
Lubinda Liswaniso gets an ultrasound as part of the sub-study on joint health for hemophilia patients.
Some bleeds are associated with a known trauma, like a twisted ankle. But for people with severe hemophilia, bleeds can be triggered by daily living activities like walking, where there’s no known injury.
“If this new medication being tested can give better protection, there’s less chance of a bleed and patients can enjoy a higher quality of life,” says Strike. “For young people, it can open the door to participating in more school and extracurricular activities, or having a part-time job with less worries of getting a bleed. By protecting the joints better, we can give young people a more normal childhood and adolescence.”
Blood in the joint is also concerning because it can lead to the development of arthropathy – joint disease — later in life which may result in the need for orthopedic surgery, adds Strike.
“Many patients with hemophilia develop advanced arthropathy at a young age, so finding a medication that provides better protection can have important lifelong implications,” she says.
HHS is recognized as one of the world’s leading health sciences research organizations. The HHS Medical Heroes series celebrates the contributions of clinical trial volunteers like Lubinda in the advancement of medical knowledge gained through studies that help with detecting, diagnosing, treating and preventing diseases. Medical Heroes stories are running throughout May, in recognition of International Clinical Trials Day on May 20.







