
HHS Medical Hero: Mackenzie Weber
A future with fewer needles
Hamilton Health Sciences (HHS) patient Mackenzie Weber can’t begin to guess the number of times he’s given blood samples, but it’s likely thousands. Now 12 years old, needles have been part of his life since he was a baby.
“I wish I didn’t have to get so much blood work done.”
Mackenzie was born with a congenital heart defect that required three surgeries when he was very young. He has taken the blood thinner warfarin for much of his life to prevent clots and is regularly monitored with blood work – meaning lots of needles.
“I wish I didn’t have to get so much blood work done,” says Mackenzie of Beamsville, who currently has monthly blood tests though there have been times in his life when this happened daily. That’s because it is difficult to get a stable drug effect with warfarin in children. The effect in a patient can fluctuate, based on diet or other medications they may be taking, so frequent monitoring with blood work is essential to ensure it’s working properly.
Joining HHS researchers
Mackenzie’s parents were asked if he would be interested in taking part in a clinical trial at HHS testing a new blood thinner for children. The drug Mackenzie tested, Edoxaban, is already approved for adults. The global study, named U313, is looking into whether this drug — which is a more stable medication and requires less monitoring in adults — is just as safe and effective as warfarin for kids.
“We’re in search of an oral blood thinner for children that requires no monitoring, but we need to make sure it behaves just as well in children as it does in adults in terms of safety and effectiveness,” says Dr. Mihir Bhatt, a pediatric hematologist and oncologist at HHS.
Bhatt, the trial’s local principal investigator, approached Mackenzie’s parents about the opportunity.
Mackenzie took part in the trial for one year, finishing in February. Clinical trials are leading to better outcomes for patients here in Hamilton, across Canada and around the world, and would not be possible without patients like Mackenzie who volunteer to take part.
Mackenzie was among 150 children worldwide selected for the trial, ranging in age from 38 gestational weeks to under 18 years old with cardiac diseases who are at risk of blood clots. For Mackenzie’s first three months in the trial, he gave blood samples once a month just as he had done when taking warfarin. Then it dropped to every three months – a welcome change from monthly needles. With his participation in the study complete, he’s back to taking warfarin, and giving monthly blood samples.
“I wanted to do the trial because I knew it would mean less blood work,” says Mackenzie, who was also motivated by the opportunity to help other children in the future if the trial is a success.
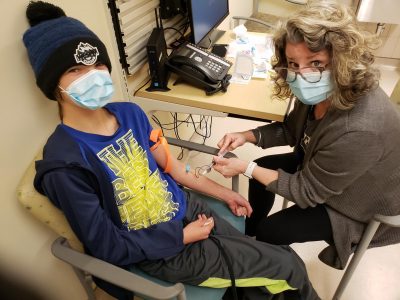
Mackenzie is pictured with Rebecca Goldsmith, clinic nurse for Thrombosis and Hemophilia.
His parents felt that the trial was safe based on conversations with Bhatt and HHS pediatric hematologist Dr. Anthony Chan. But they also had to consider logistics, like whether it would mean additional visits to the hospital since they are both teachers and don’t have a lot of flexibility for daytime appointments.
“We talked to Drs. Bhatt and Chan about the reasoning behind the study, and felt that it could potentially help Mackenzie as well as other children in the future,” says Mackenzie’s mom Adriana Weber.
Improving the quality of life for children
“It’s very important that these children are put on blood thinners long-term to prevent blood clots,” says Bhatt.

Dr. Mihir Bhatt
But options for children are limited. Currently the main option is warfarin, in pill form. The second, and far less popular option, is a medication requiring an injection twice daily. “You can imagine how challenging this is for a child in terms of pain and anxiety,” says Bhatt.
Once the trial is complete, results will be analyzed and published. If it’s a success, the company running the study would then apply to Health Canada for approval. If approved, it could be available to Canadian children in an estimated two to five years.
“We would love to find a safe and reliable alternative for Mackenzie and other children with this condition.”
“It’s a step in the right direction,” says Adriana. “Any parent in our situation would welcome the opportunity for their child to have a more stable medication with less frequent blood work. Needles are painful, and no one wants to see their child suffer. Warfarin is so unreliable at times, and so inconsistent. We would love to find a safe and reliable alternative for Mackenzie and other children with this condition.”
HHS is recognized as one of the world’s leading health sciences research organizations. The HHS Medical Heroes series celebrates the contributions of clinical trial volunteers like Mackenzie and his family in the advancement of medical knowledge gained through studies that help with detecting, diagnosing, treating and preventing diseases. Medical Heroes stories are running throughout May, in recognition of International Clinical Trials Day on May 20.







